Many genetic disorders are caused by faulty versions of a single gene. In the last decade, scientists have made tremendous strides in correcting these faults through “gene therapy”—using viruses to sneak in working versions of the affected genes.
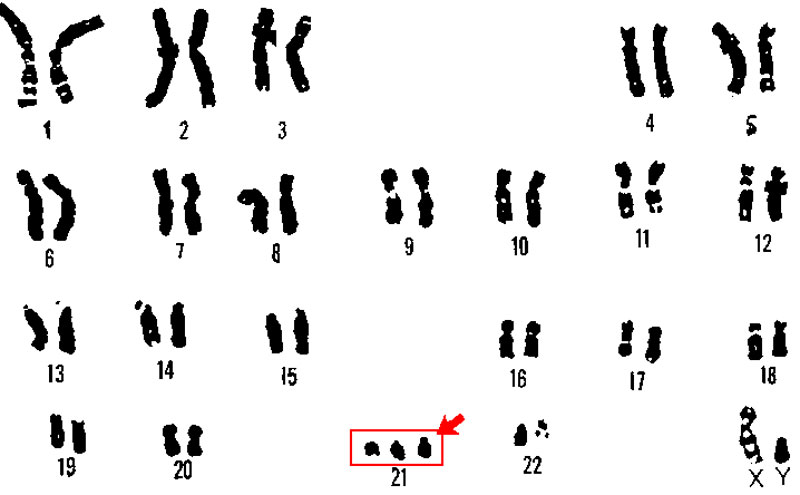
But some disorders pose greater challenges. Down’s syndrome, for example, happens when people are born with three copies of the 21st
chromosome, rather than the usual two. This condition, called trisomy,
leads to hundreds of abnormally active genes rather than just one. You
cannot address it by correcting a single gene. You’d need a way of
shutting down an entire chromosome.
But half of us do that already. Women are masters of chromosomal silencing.
Women are born with two copies of the X chromosome, while men have
just one. This double dose of X-linked genes might cause problems, so
women inactivate one copy of X in each cell.
This is the work of a gene called XIST (pronounced “exist”). It
produces a large piece of RNA (a molecule closely related to DNA) that
coats one of the two X chromosomes and condenses it into a dense,
inaccessible bundle. It’s like crunching up a book’s pages to make them
unreadable and useless. XIST exists on the X chromosome, so that’s what
it silences. But it should be able to shut down other chromosomes too,
if we could just insert it into the right place.
That’s exactly what Jun Jiang
from the University of Massachusetts Medical School has done: she used
XIST to shut down chromosome 21. “Most genetic diseases are caused by
one gene, and gene therapies correct that gene,” says Jeanne Lawrence,
who led the study. “In this case, we show that you can manipulate one
gene and correct hundreds.” It’s chromosome therapy, rather than gene
therapy.
So far, the team have only done this in Down’s syndrome cells, grown
in a laboratory, so the technique is a very long way from any clinical
use. But it’s a promising first step, and other scientists are very
excited. “It’s an amazing paper,” says Elizabeth Fisher
from University College London, who studies Down’s syndrome. “The fact
that they have silenced the entire chromosome will really help people to
dissect what’s going wrong in Down’s syndrome.”
High-risk, high-reward
Lawrence has spent years studying XIST, and has always thought about
applying this work to Down’s syndrome. After all, she used to provide
counselling for parents whose babies are born with disabilities, and she
regularly talks to families who are affected by Down’s, some of whom
talk at the genetics course she runs. But while using XIST to inactivate
chromosome 21 was an obvious strategy, it was also a risky one.
For a start, XIST is huge—far larger than any other gene that
has been deliberately inserted into a genome before. If the team got it
into the right place, would it actually silence chromosome 21 without
killing the cell? And if it worked, what would stop it from silencing all three copies
rather than just one? “None of these challenges made the project
impossible, but collectively they made it pretty improbable,” says
Lawrence. “We didn’t know if we’d spend years not getting anything to
work.”
And yet, after six years of toil, it worked. Jiang used enzymes
called zinc finger nucleases, which cut DNA at very specific points, to
smuggle the giant XIST gene into a pre-defined spot on the 21st chromosome. She did this in cells from a boy with Down’s syndrome, which had been reprogrammed into a stem cell-like state.
XIST did its thing, “painting” one of the three chromosome-21s, and
condensing it into a tight bundle. The genes on that copy were almost
totally inactivated.
In this study, Jiang ensured that XIST only shut down one of the
three chromosomes by tweaking its concentration. In the future, the team
might target it to sequences found in only one of the three copies.
But does inactivating a copy of chromosome 21 achieve anything
useful? Jiang saw some promising signs. For example, after XIST, the
Down’s cells grew more quickly, produced larger colonies, and were far
better at dividing into neuron-making cells. This supports the idea that people with Down’s syndrome can’t make enough cells (and neurons, in particular) as they grow up.
Benefits
“It’s an extremely exciting development. It’s somewhat surprising
that it took so long for someone to apply this to chromosome 21, but the
group had to overcome some very significant technical challenges,” says
Roger Reeves
from Johns Hopkins University. “The next step will be to silence an
extra chromosome in an animal, as opposed to a dish of cells.” For
example, they could try the technique on mice that have been bred with
extra copies of chromosome 21.
Even if that worked, it would be very challenging to use the XIST
technique in people—you’d need to get the giant gene into the right
cells at the right stage. “I doubt that XIST by itself has the
potential to become a therapeutic agent in patients,” says Stylianos Antonarakis from the University of Geneva.
Lawrence agrees, but she thinks there might be exceptions. For example, many children with Down’s develop myoproliferative disease,
where they produce too many blood cells and run a high risk of
leukaemia. If doctors saw kids with this condition, it might be
possible to activate XIST in their blood stem cells, to prevent them
from developing cancer. “That’s one of the more likely possible uses,”
says Lawrence.
The study also has more immediate benefits: “It’s a way of getting at
the biology that underlies the different aspects of Down’s,” says
Lawrence. The syndrome includes dozens of symptoms across many different
organs, including intellectual disabilities, heart problems, leukaemia
and Alzheimer’s at an early age. Matching these up to the hundreds of
genes on chromosome 21 has been a herculean task. “There are many
studies that point to different genes but it’s still a pretty confused
field,” says Lawrence.
Her team’s work could help. Scientists could activate XIST in one of
two groups of identical cells, and watch what happens to the rest of
their genes. They could do this in neurons, heart cells, or any of the
other tissues that are affected in Down’s syndrome. They could also test
drugs that are designed to alleviate the syndrome’s symptoms. And, as
Antonarakis says, scientists could do this not just for Down’s syndrome,
but for the many other disorders that are caused by unusual number of chromosomes.
Jiang’s work also confirms something important about XIST—it evolved
to shut down the X chromosome, but it works on all of them. “It must be
acting on something that’s found on all chromosomes,” says Lawrence. She
thinks it might recognise repetitive bits of DNA that are found
throughout our genome, but have no obvious purpose.
Indeed, Lawrence suspects that her work on XIST and Down’s might
eventually tell us more about how the genome is organised. XIST is one
of several pieces of RNA that are transcribed from the genome, but never
used to make proteins. Because of its large size, it’s classified as a
“long, non-coding RNA” or lncRNA—a group that includes tens of thousands of members.
A minority of these, like XIST, clearly help to control how other genes
are used, but there’s a lot of debate about what the rest do, if
anything (see Carl Zimmer’s post for more).
Lawrence’s team have moved beyond this debate, and are one of the
first to actually use a lncRNA to target and silence a set of genes.
“That’s one of the aspects that makes it so exciting,” says Mitchell Guttman from the California Institute of Technology, who studies lncRNA and recently showed how XIST finds its way around the X chromosome.
“The field will surely build upon this in the future as it continues to
dissect the roles of other lncRNAs and learns more about the principles
governing their localization and function.”
Reference: Jiang, Jing, Cost, Chiang, Kolpa, Cotton, Carone,
Carone, Shivak, Guschin, Pearl, Rebar, Byron, Gregory, Brown, Urnov,
Hall & Lawrence. 2013. Translating dosage compensation to trisomy
21. Nature
Source :


0 comments:
Speak up your mind
Tell us what you're thinking... !